by PEPID Newsroom | Jul 23, 2019 | Alerts
A data analysis of 9 studies from 7 publications totaling to 307, 099 participants with 23,544 cases of incident type 2 diabetes showed a significant inverse association between people who adhered to a plant-based dietary pattern and risk of type 2 diabetes compared...
by PEPID Newsroom | Jul 23, 2019 | Alerts
Low-dose aspirin has been recommended and used for decades for prevention of ASCVD. It is well established for SECONDARY prevention of ASCVD, however, recent studies have shown aspirin should not be used for routine PRIMARY prevention of ASCVD due to lack of benefit....
by PEPID Newsroom | Jul 11, 2019 | Alerts
New study shows C-reactive protein (CRP) testing in primary care clinics for patients with Chronic Obstructive Pulmonary Disease (COPD) reduces patient-reported antibiotic use or receiving antibiotic prescriptions for exacerbation of COPD with no evidence of harm....
by PEPID Newsroom | Jul 10, 2019 | Alerts
According to the NutriNet-Sante prospective cohort study of 101,257 participants with the mean age of 42 and a medium follow-up of 5 years, sugary drinks like soda and fruit juices show a significant association of risk of overall cancer, and breast cancer....
by PEPID Newsroom | Jul 9, 2019 | Alerts
According to a report published in the Annals of Internal Medicine by the American College of Physicians, there is no benefit from antibiotic treatment exceeding the shortest effective duration when treating hospitalized patients with pneumonia. Excess days were...
by PEPID Newsroom | Jul 8, 2019 | Alerts
In a report published July 8, 2019 in JAMA Network, dispensing oral contraceptive pills 12-months rather than 3-months saves money and provides better support for women’s reproductive goals and autonomy. The study was done to estimate the financial and reproductive...
by PEPID Newsroom | Jul 2, 2019 | Alerts
A recent study published in the American Academy of Neurology observed a significant association between low-density lipoprotein cholesterol (LDL-C) concentrations and intracerebral hemorrhage (ICH) risk. The current cohort study included 96,043 participants with the...

by PEPID Newsroom | Jun 21, 2019 | Alerts
FDA approves bremelanotide (Vyleesi, AMAG Pharmaceuticals) to treat acquired, generalized hypoactive sexual desire disorder (HSDD) in premenopausal women. Bremelanotide is the first-in-class melanocortin 4 receptor agonist for HSDD, and joins Flibanserin as...

by PEPID Newsroom | Jun 4, 2019 | Alerts
Emgality (gacanezumab-gnlm) solution has been FDA approved for treatment of episodic cluster headache in adults, the first of its kind. Emgality was approved in 2018 to prevent migraines in adults, and now has shown to reduce the frequency of cluster headache attaches...

by PEPID Newsroom | Jun 4, 2019 | Alerts
The U.S. FDA approved a new indication for Zerbaxa (Ceftolozane and tazobactam) for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABD/VABP) in patients 18 years and older. This approval addresses the threat of...

by PEPID Newsroom | May 24, 2019 | Alerts
Pigray (alpelisib) tablets have been FDA approved to treat breast cancer in combination with FDA-approved endocrine therapy fulvestrant. Pigray is approved for postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2...

by PEPID Newsroom | May 23, 2019 | Alerts, Pharmacy
Every year, the US FDA’s Center for Drug Evaluation and Research (CDER) releases a list of newly-approved novel drugs and therapeutic biological products. Last year’s Novel Drug Approvals list holds the record for the most number of approved drugs, effectively...

by PEPID Newsroom | May 20, 2019 | Alerts
The American Stroke Association released improved recommendations for stroke systems of care to improve patient outcomes. It aims to extensively review of the current evidence evaluating these systems, and provide updates for policymakers and public healthcare...
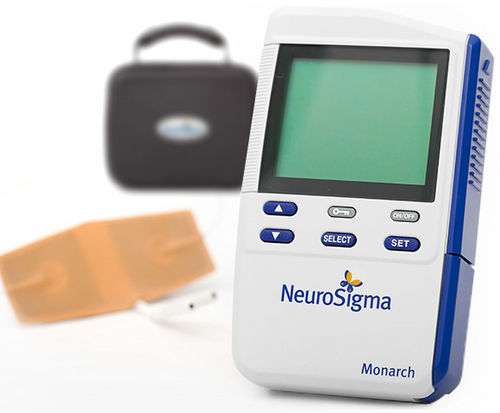
by PEPID Newsroom | Apr 19, 2019 | Alerts
Children living with attention hyperactivity disorder (ADHD) may soon experience non-pharmacologic relief through a breakthrough medical device. For children between the ages 7 and 12 and currently not on medication, the pocket-sized device works by delivering mild...
by PEPID Newsroom | Apr 18, 2019 | Alerts
Update to PEPID Pulse “E. Coli O103 Outbreak Investigation in Five States” on April 5 , 2019 The CDC has narrowed the Shiga Toxin-producing Escherichia Coli (E. Coli) O103 outbreak to ground beef. The total number of people infected has increased to 109 people...







