
by PEPID Newsroom | May 24, 2019 | Alerts
Pigray (alpelisib) tablets have been FDA approved to treat breast cancer in combination with FDA-approved endocrine therapy fulvestrant. Pigray is approved for postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2...
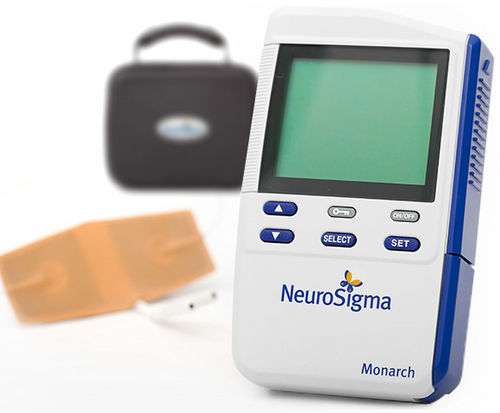
by PEPID Newsroom | Apr 19, 2019 | Alerts
Children living with attention hyperactivity disorder (ADHD) may soon experience non-pharmacologic relief through a breakthrough medical device. For children between the ages 7 and 12 and currently not on medication, the pocket-sized device works by delivering mild...
by PEPID Newsroom | Mar 14, 2019 | Alerts
As of March 12, 2019 the U.S. FDA approved a new generic of Diovan (valsartan) for high blood pressure and heart failure to relieve the shortage from multiple recalls of generic valsartan products from several manufacturers. The approval of the new generic of Diovan...




