Moving Forward with Proper PGx Expectations 2,5
Pharmacogenomic (PGx) testing and PGx-based treatments may be all the rage in precision medicine today, but more genetic and pharmacology experts – especially in the areas of cardiology, pain management, and psychiatry – are speaking up and pumping the brakes. With commercial PGx testing entities boldly touting their results as the key to finding the perfect drug, do providers and patients really know what pharmacogenomics testing can and cannot reveal? As pharmacogenomics joins the era of Big Data, clinicians should be prepared to understand, explain, and respond to the increasing number of patients walking in with pharmacogenomic test results. These patients have likely spent at least $200 and 7-10 days waiting on their color-coded, warning-riddled paper. So, not only are they now walking into your office with results, but with more questions, and more misunderstandings of phrases such as “use with increased caution and more frequent monitoring.” If clinicians don’t take the time to set expectations and clarify sweeping marketing statements from commercial testing providers, the cycle of patient mistrust and nonadherence will perpetuate.
Moving Forward with the Facts 3
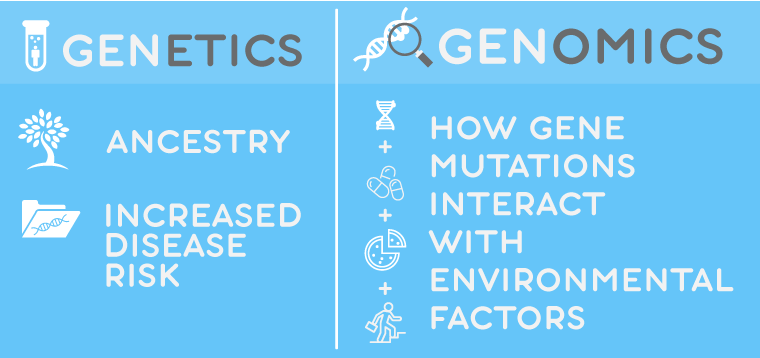
1. PGx can’t explain eye color or predict disease risk
Start the conversation by clarifying how genetic testing is different from genomic testing. Genetics can trace your ancestry and analyze how genes are expressed, including inherited disease risk. Genomics, on the other hand, studies how specific mutations interact with environmental factors, such as pharmacologic treatments. Even though studies remain few, most of today’s commercially available PGx testing revolves around pharmacokinetics (how the body metabolizes the drug). For example, a test can reveal gene variations that affect how cytochrome P450 (CYP) enzymes are expressed. A patient with an ultra-rapid metabolizer may simply need a higher dose or extended release formulation to achieve therapeutic levels – not necessarily a different drug. Pharmacodynamic findings are even fewer. An example would be an FDA Black Box warning to test a patient of Asian descent for an HLA-B1502 variant linked with Steven-Johnson Syndrome – a systemic ADR to carbamazepine, common in patients of Asian descent.
2. PGx does not predict patient response to all drugs on the market
Current research is focused on drugs in the realm of cardiology, pain management, and psychiatry. There are many other drugs that have yet to be researched.
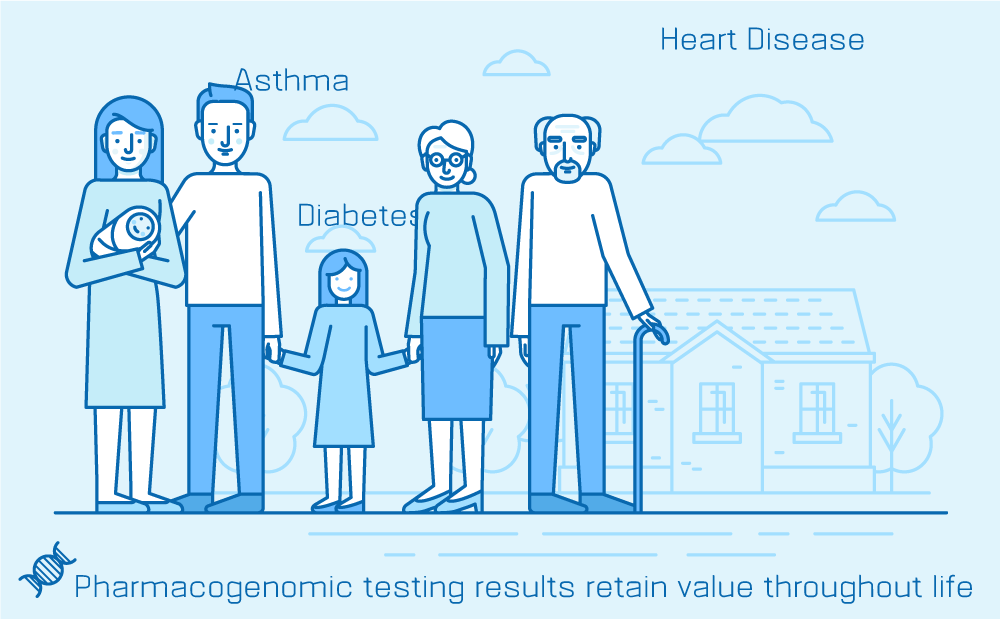
3. PGx results retain value throughout life
This is where clinicians can encourage proactive PGx testing for application in future care.
4. PGx is more relevant to discuss with non-responders
Pharmacologic trials and errors are costly and discomforting for any patient. Patients who’ve had a streak of unsatisfactory treatment responses benefit more from the peace of mind pharmacogenomics results provide, especially prior to starting a new regimen.
5. PGx can be enhanced with goal-setting
Because pharmacogenomics offers a lifetime of insight, goal-setting in the face of chronic disease and familial history adds even more value to results. For example, a patient who reports a familial history of heart disease will benefit from a test that includes variants linked to antilipidemics and blood thinners. Plavix, for example, carries a Black Box Warning for patients with the CYP2C19 liver enzyme, as a normal dose for these patients could cause a hemorrhage or stroke.
6. PGx cannot pinpoint that one perfect drug
There remains a scarcity of independent, randomized, controlled trials that confirm the benefits of pharmacogenomic-informed prescribing. Any easily-searched publication should be taken with a grain of salt as most are funded and analyzed by commercial PGx providers and draw conclusions from small sample sizes.
7. PGx testing may simply rule out genetic factors 4
PCPs often order pharmacogenomic testing to rule out genetic makeup as a reason for a patient’s suboptimal treatment response. In this case, the provider-patient tandem can investigate other physiological and environmental factors, such as:
- Age
- Gender
- Ethnic differences
- Diet
- Hormone balance
- Impaired liver
- Kidney function
- Drug to Drug interactions
- Drug to Food interactions
More experts are starting to agree that even though promoting PGx is beneficial, clinicians must remember to correct patient misconceptions that these results can determine which drugs will and will not work. Commercial testing entities should exercise more responsible marketing claims when it comes to pharmacogenomics, and give PCPs room to offer interpretations and recommendations that go beyond theoretical evidence.
[elfsight_social_share_buttons id=”3″]
>> Join the Movement at ASHP Midyear 2018
If You’re Considering Implementing RPM, Consider PEPID
Meet Us at Booth #513
Resources:
- Phillips, K A, et al. “Potential Role of Pharmacogenomics in Reducing Adverse Drug Reactions: a Systematic Review.” , U.S. National Library of Medicine, 14 Nov. 2001, http://www.ncbi.nlm.nih.gov/pubmed/11710893/.
- Hussain, S et al. “Disease-drug database for pharmacogenomic-based prescribing” Clinical pharmacology and therapeutics vol. 100,2 (2016): 179-90.
- Dunnenburger, Mark. “7 Things to Know About Pharmacogenomics.” S. News & World Report, U.S. News & World Report, health.usnews.com/health-news/patient-advice/articles/2016-08-01/7-things-to-know-about-pharmacogenomics.
- Bailey, K. “Physiological Factors Affecting Drug Toxicity.” Regulatory Toxicology and Pharmacology : RTP., U.S. National Library of Medicine, Dec. 1983, https://www.ncbi.nlm.nih.gov/pubmed/6658034.
- Fan, Jianqing and Han Liu. “Statistical analysis of big data on pharmacogenomics” Advanced drug delivery reviews 65,7 (2013): 987-1000.

