On July 20, 2018, the U.S. Food and Drug Administration (FDA) approved the first acute myeloid leukemia treatment. It is the first approved targeted cancer therapy for patients with relapsed or refractory acute myeloid leukemia (R/R AML), specifically, those screened by a complementary FDA-approved test and found to be carrying a mutated isocitrate dehydrogenase-1 (IDH1) gene. This makes wholly-owned Tibsovo (ivosidenib) the first in its class of IDH1-inhibitors, and the second drug from Agios Pharmaceuticals, Inc.’s pipeline to successfully land on the market.
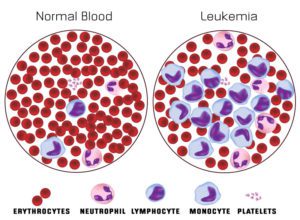
Normal Blood Smear vs Acute Myeloid Leukemia Blood Smear
This is good news for about 6-10% of AML patients carrying this specific mutation who now have an acute myeloid leukemia treatment option. This precision medicine can greatly improve patients’ quality of life during therapy, markedly improves patient outcomes, and is an alternative treatment option for patients who cannot tolerate traditional anticancer therapy. 1,2,3
At present, the Leukemia & Lymphoma Society (LLS) estimates that there are 381,774 people in the U.S. who are either still battling leukemia or are in remission from it. AML is a particularly concerning form of leukemia in adults, as it progresses rapidly and has a 27.4% survival rate overall – the lowest number among all types of leukemia. In fact, it is estimated that in 2018 alone, 19,520 people will be diagnosed with AML, and about 10,670 AML patients will die from it. 1
Director of the FDA’s Oncology Center of Excellence and acting Director of the Office of Hematology and Oncology Products, Dr. Richard Pazdur, shares the agency’s excitement in welcoming Tibsovo to the market as a targeted solution. Patients can now avail of an acute myeloid leukemia treatment option that has been associated with complete remission and lessens the likelihood of needing blood transfusions. 1
The study that earned Agios Pharma the FDA approval was an open-label, single-arm, multicenter dose-escalation and expansion trial that enlisted 174 adults with R/R AML who were screened for the IDH1 mutation using the FDA-approved Abbott RealTime™ IDH1 companion diagnostic test. Subjects had undergone an average of 2 anticancer therapies prior to being given Tibsovo 500mg tablets daily during the study. Results showed a combined complete remission (CR) and complete remission with partial hematologic improvement (CRh) rate of 32.8% (57 of 174 subjects) that lasted an average of 8.2 months. 2,5
Sources:
- “FDA Approves First Targeted Treatment for Patients with Relapsed or Refractory Acute Myeloid Leukemia Who Have a Certain Genetic Mutation.” Food and Drug Administration, 20 July 2018, www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm614115.htm.
- “FDA Grants Approval of TIBSOVO®, the First Oral, Targeted Therapy for Adult Patients with Relapsed/Refractory Acute Myeloid Leukemia and an IDH1 Mutation.” Agios Pharmaceuticals, Inc., 20 July 2018, agios.com/news-releases/news-release-details/fda-grants-approval-tibsovor-first-oral-targeted-therapy-adult.
- Gerber, David E. “Targeted Therapies: A New Generation of Cancer Treatments.” American Family Physician, 1 Feb. 2008, org/afp/2008/0201/p311.html.
- “Facts and Statistics.” Leukemia and Lymphoma Society, 3 Mar. 2015, org/node/22231#Leukemia.
- “Abbott RealTime IDH1.” Abbott Molecular, www.molecular.abbott/us/en/products/oncology/realtime-idh1.