
by Megan Pullins | Jul 16, 2018 | Emergency Medicine, Nursing, Pharmacy, Primary Care
Evidence that Adverse Drug Interactions Occur from Herbal-Drug Interactions and Non-Prescription Drug Interactions. “Natural” is widely perceived by the public to ensure safety. However, studies show drug interactions with active pharmaceutical agents, and...

by Megan Pullins | Apr 16, 2018 | Emergency Medicine
In 2017, the US had 16 natural disasters. Drought, wildfires, flooding, freezing, severe weather, tornadoes, and three hurricanes. Hurricane Harvey affected 13 million people from Texas to Kentucky. 300 volunteer organizations stepped up to help. Hurricane...

by Alex Urbanczyk | Jul 16, 2024 | Featured Posts
To achieve the highest level of success, nurses must feel compelled to perform compassion toward patients. Compassionate care is the cornerstone of nursing practice, reflecting nurses’ deep empathy and genuine concern for their patient’s well-being. It goes beyond the...

by Charles Choate | Jun 7, 2024 | Uncategorized
Wearable technology has swiftly become an integral part of modern healthcare, offering innovative solutions to monitor and manage patient health. Devices such as smartwatches, biosensors, and wearable ECG monitors are transforming the landscape of medical care,...

by Charles Choate | May 13, 2024 | Uncategorized
(Children) present with a unique array of physiological and psychological attributes that necessitate a specialized approach to emergency medical treatment. In the fast-paced realm of emergency medicine, the care of pediatric patients stands out as a particularly...

by Charles Choate | Apr 17, 2024 | Uncategorized
In such a high-pressure environment, having access to reliable and concise information is not just helpful—it’s essential. Emergency medical services (EMS) professionals navigate a challenging landscape where quick, accurate decisions are crucial. The risk of...

by Erin Grim | Feb 12, 2024 | Uncategorized
Listen to the Narrated version 18min https://blog.pepid.com/wp-content/uploads/2024/03/CDS-blog_mixdown.mp3 Clinical Decision Support apps have the potential to transform the way healthcare professionals approach patient care, by providing real-time access to...

by Charles Choate | Feb 12, 2024 | Uncategorized
Disaster preparedness involves preparing emergency departments and healthcare facilities to provide medical care to a large number of patients with diverse medical needs during a disaster. Disaster preparedness in emergency medicine encompasses the strategic process...

by Charles Choate | Jan 11, 2024 | Uncategorized
Good bedside manner is not always enough to effectively manage all patients in the Emergency Department Patients in the emergency department (ED) can sometimes present a spectrum of challenges for the healthcare team, ranging from uncooperative or combative behaviors...
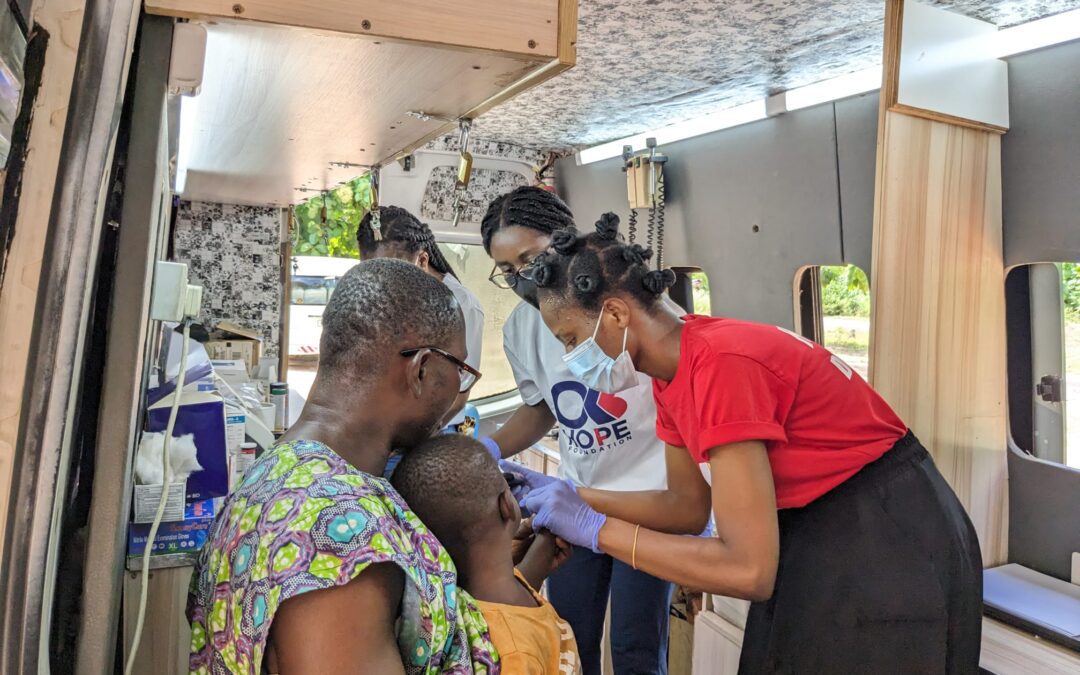
by Charles Choate | Dec 14, 2023 | Uncategorized
About OKB Hope Foundation: The OKB Hope Foundation stands as a testament to the power of dedicated healthcare service in rural and underserved communities. With a mission to bring quality healthcare to the doorsteps of those in remote areas, the foundation has been a...

by Erin Grim | Sep 11, 2023 | Uncategorized
Drug overdoses have become a significant public health crisis, with devastating consequences for individuals, families, and communities. Alarming data from the World Health Organization (WHO) underscore the gravity of the situation, indicating that drug overdoses are...

by Erin Grim | Sep 11, 2023 | Uncategorized
The field of emergency medicine is in a constant state of evolution, with technological advancements playing an increasingly pivotal role in enhancing patient care. One such technology is virtual reality (VR), which is being used to revolutionize emergency medical...













